Background: The potent and selective oral menin-histone-lysine N-methyltransferase 2A (KMT2A) inhibitor revumenib (SNDX-5613) induced complete remissions with clearance of residual disease in acute leukemias refractory to multiple previous lines of therapy ( Nature. 2023;615:920-924). Here we report outcomes following the resumption of revumenib after hematopoietic stem cell transplant (HSCT).
Methods: Patients aged ≥30 days with relapsed or refractory (R/R) acute leukemias with genetic alterations associated with HOXA overexpression, including KMT2A-rearranged ( KMT2Ar) or nucleophosmin 1 ( NPM1)-mutated leukemias, were enrolled in the AUGMENT-101 phase 1/2, multicenter, open-label, dose-escalation study (NCT04065399). Patients received revumenib every 12 hours (q12h) in 28-day continuous cycles. Patients who achieved a complete response (composite definition [CRc]), morphological leukemia-free state, or partial response could undergo HSCT without leaving the study. Revumenib was stopped before the HSCT conditioning regimen but, per a protocol amendment, could be resumed after HSCT if the patient was in CRc. Measurable residual disease (MRD) was assessed using multiparameter flow cytometry and/or polymerase chain reaction.
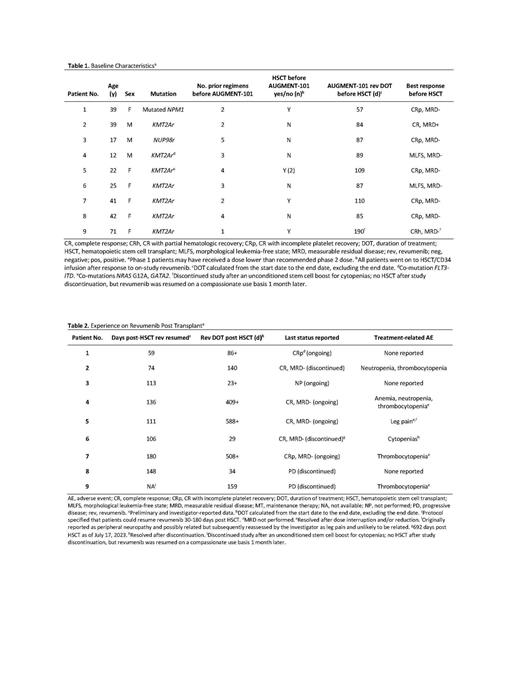
Results: Between November 5, 2019, and June 28, 2023, a total of 131 phase 1 patients were enrolled and treated in the AUGMENT-101 study. Nine patients with acute myeloid leukemia (AML) from the AUGMENT-101 study resumed revumenib (8 after transplant and 1 after a stem cell boost [Table 1]). Seven patients had KMT2Ar and 1 patient each had nucleoporin 98 rearranged (NUP98r) and mutated NPM1. Revumenib was resumed 59 to 180 days after HSCT (Table 2). Dose was reduced for 4 of 9 patients to mitigate adverse events of thrombocytopenia (n=3) and leg pain (n=1), and revumenib was discontinued in 4 of 9 patients because of progressive disease (n=2), cytopenias (n=1), and not reported (n=1). Revumenib duration of treatment (DOT) in the maintenance setting at the time of this analysis ranged from 23 to 588 days, with treatment ongoing for 5 of the 9 patients. CRc was maintained in 6 of 9 patients after HSCT and maintenance revumenib. One patient with reported MRD after HSCT converted to MRD-negative status following initiation of revumenib maintenance therapy. Overall, MRD-negative remissions were maintained in 5 patients as of the data cut off.
Conclusions: With 3 patients on revumenib maintenance therapy for more than a year, long-term responses, including conversion to MRD-negative status, were seen in these heavily pretreated patients with AML. Resuming revumenib post HSCT had a tolerable safety profile consistent with that previously reported for the AUGMENT-101 study.
Disclosures
Zucenka:Abbvie: Consultancy, Honoraria, Other: Travel Expenses; Astellas: Consultancy, Honoraria; Pfizer: Consultancy; Novartis: Consultancy, Honoraria, Other: Travel Expenses; Jannsen: Consultancy, Honoraria, Other: Travel Expenses; Takeda: Other: Travel expenses. Issa:Kura Oncology: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; NuProbe: Consultancy; Merck: Research Funding; Celgene: Research Funding; Syndax: Research Funding. Khera:Incyte: Honoraria. Stock:Glaxo Smith Kline: Consultancy; Servier: Other: Data Safety Monitoring Board/Advisory Board; Newave: Honoraria; Kite: Consultancy; Jazz Pharmaceuticals: Consultancy, Honoraria; Kura: Research Funding; Amgen: Honoraria. Gu:Syndax Pharmaceuticals: Current Employment, Current holder of stock options in a privately-held company. Nguyen:Syndax Pharmaceuticals: Current Employment, Current holder of stock options in a privately-held company. Smith:Syndax Pharmaceuticals: Current Employment, Current holder of stock options in a privately-held company. Stein:Ono Pharma: Consultancy; OnCusp: Consultancy; Neoleukin: Consultancy; Agios: Consultancy; Syndax: Consultancy; Menarini: Consultancy; Janssen: Consultancy; Jazz: Consultancy; Syros: Consultancy; Gilead: Consultancy; Aptose: Consultancy; Daiichi: Consultancy; Calithera: Consultancy; Servier: Consultancy; Foghorn: Consultancy; CTI Biopharma: Consultancy; Abbvie: Consultancy; Genesis: Consultancy; Genentech: Consultancy; PinotBio: Consultancy; Novartis: Consultancy; Bristol Myers Squib: Consultancy, Research Funding; Eisai: Research Funding; Blueprint: Consultancy; Astellas: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal